
Leader: Deric Wheeler, PhD
Associate Professor
Human Oncology
University of Wisconsin

Co-leader: Randall Kimple, MD, PhD
Radiation Oncologist
Associate Professor
Human Oncology
University of Wisconsin
Summary:
The only approved molecular targeting agent in the treatment of Head and Neck Cancer (HNC) is cetuximab (CTX), an antibody directed against the epidermal growth factor receptor (EGFR). The vast majority of patients are either intrinsically resistant or acquire resistance to this promising agent. We have identified the receptor tyrosine kinase AXL as a major driver of cetuximab resistance. We will investigate how AXL mediates resistance, whether AXL blockade can re-sensitize resistant tumors and if AXL can serve as a biomarker to predict cetuximab resistance in HNC.
Specific Aims:
-
AIM 1: Determine A) how AXL activates the HER3/PI3K/Akt pathway in Head and Neck Cancer Squamous Cell Carcinoma (HNSCC) and B) if AXL mediated activation of the HER3/PI3K/Akt pathway leads to CTX resistance.
-
AIM 2: Determine if targeting AXL in mouse models of CTXR can enhance and re-sensitize tumors to CTX.
-
AIM 3: Determine A) whether AXL predicts resistance to CTX in HNSCC patients and B) if targeting AXL in CTXR patient-derived xenografts (PDX) enhances tumor response to CTX therapy.
Publications:
-
Cotargeting mTORC and EGFR Signaling as a Therapeutic Strategy in HNSCC Molecular cancer therapeutics
Swick AD, Prabakaran PJ, Miller MC, Javaid AM, Fisher MM, Sampene E, Ong IM, Hu R, Iida M, Nickel KP, Bruce JY, Wheeler DL, Kimple RJ
2017 Jul;16(7):1257-1268. doi: 10.1158/1535-7163.MCT-17-0115. Epub 2017 Apr 26.
-
More
Head and neck squamous cell carcinomas (HNSCC) are frequently altered along the PI3K/AKT/mTORC signaling axis. Despite excellent preclinical data, the use of compounds targeting this pathway as monotherapy has been underwhelming in initial clinical trials, and identification of predictive biomarkers remains challenging. To investigate mTORC-specific inhibition, we tested catalytic mTORC (AZD8055) and PI3K/mTORC (NVP-BEZ-235) inhibitors ± cetuximab in a panel of HNSCC cell lines and patient-derived xenografts (PDX). Cell lines were assayed for response to all agents and siRNA knockdown of targets by multiple approaches. All cell lines showed similar response to both drug and siRNA inhibition of both PI3K and mTORC pathways, with anti-EGFR combination producing modest additive effect. Five PDX models that presented PIK3CA mutation or intrinsic cetuximab resistance were treated with a combination of cetuximab and AZD8055. In vivo single-agent mTORC inhibition inhibited growth of one PIK3CA-mutant cancer, but had little effect on any PIK3CAWT or a second PIK3CA-mutant model. In all models, the combination therapy showed greater growth delay than monotherapy. The uniform ability of PI3K and mTORC inhibition to suppress the growth of HNSCC cells highlights the pathway's role in driving proliferation. Although single-agent therapy was largely ineffective in vivo, improved response of combination treatment in an array of PDXs suggests the potential for adding a catalytic mTORC inhibitor to cetuximab therapy. Overall, these results add to a growing body of evidence, suggesting that approaches that attempt to match biomarkers to the optimal therapy in HNSCC remain complex and challenging. Mol Cancer Ther; 16(7); 1257-68. ©2017 AACR.
PMID:28446642 | PMC:PMC5505754 | DOI:10.1158/1535-7163.MCT-17-0115
View details for PubMedID 28446642
-
More
-
Cotargeting mTORC and EGFR Signaling as a Therapeutic Strategy in HNSCC Head and neck squamous cell carcinomas (HNSCC) are frequently altered along the PI3K/AKT/mTORC signaling axis. Despite excellent preclinical data, the use of compounds targeting this pathway as monotherapy has been underwhelming in initial clinical trials, and identification of predictive biomarkers remains challenging. To investigate mTORC-specific inhibition, we tested catalytic mTORC (AZD8055) and PI3K/mTORC (NVP-BEZ-235) inhibitors ± cetuximab in a panel of HNSCC cell lines and...
-
More
View details for PubMedID pubmed:28446642
-
More
Collaborator:

Justine Yang Bruce, MD
Medical Oncologist
Associate Professor of Medicine
University of Wisconsin
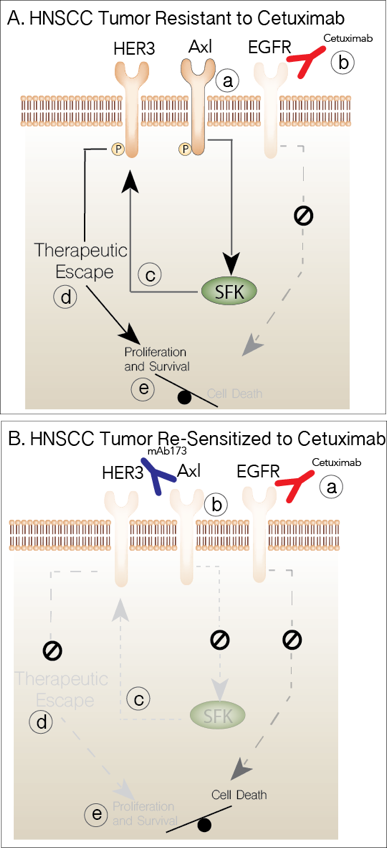
Fig. 1. Targeting AXL Overcomes CTXR. A) Tumors resistant to CTX overexpress AXL (a). Inhibition of EGFR blocks signals to proliferation and survival (b). AXL activates the SFKs (c), which in turn play a role in activating HER3 mediating therapeutic resistance (d) and maintaining proliferative and survival signals (e). B) Collectively, blocking EGFR (a) and AXL (b) leads to decreased SFK (c) and HER3 (d) activity and blocks all signals tilting the balance to cell death.
Learn more:
Head and Neck Squamous Cell Carcinoma (HNSCC) is the sixth most common cancer worldwide with more 600,000 new cases diagnosed annually. In the United States alone, there are approximately 56,000 newly diagnosed HNSCC patients each year with more than 12,000 associated deaths. Treatment for HNSCC patients includes surgery, radiation and chemotherapy in various combinations. Cure rates have improved only marginally over the last 30 years. The most promising new drug advance has been the 2006 FDA approval of cetuximab (CTX), a monoclonal antibody against the epidermal growth factor receptor (EGFR) in the treatment of HNSCC. Addition of CTX to standard radiation improves absolute survival rates for newly diagnosed HNSCC patients by approximately 10 percent and is also valuable in the metastatic/recurrent setting where approximately 36 percent of patients experience response to CTX based combination therapy (vs. 20 percent to standard therapy). Although CTX offers a significant advance for many patients with HNSCC, nearly two-thirds of EGFR expressing patients do not respond to CTX-based therapy. Virtually all patients who initially respond, eventually become refractory to CTX. Intrinsic and acquired resistance to CTX is therefore a significant clinical problem in the treatment of HNSCC.
To identify drivers of CTX resistance (CTXR) in HNSCC, our lab has utilized HNSCC human tumor specimens, patient-derived xenografts (PDXs) and a series of acquired and intrinsically resistant in vitro and in vivo model systems. Investigation of these models to isolate molecular mechanisms of CTXR has generated several significant findings:
- Analysis of a 63 HNSCC patient cohort revealed that the receptor tyrosine kinase AXL was overexpressed in both human papillomavirus (HPV)-positive and HPV-negative HNSCC tumors and was significantly associated with higher pathologic grade, distant metastases and shorter relapse-free survival.
- Cetuximab-resistant (CTXR) HNSCC PDXs, relative to CTX-sensitive (CTXS) tumors, expressed significantly elevated levels of total and activated AXL.
- In vitro and de novo (in vivo) models of acquired resistance showed increased expression and activity of AXL.
- Models of intrinsically resistant HNSCC lines showed increased AXL expression and activity.
- Genetic ablation or therapeutic inhibition (antibody or small molecule inhibitors) of AXL led to increased sensitivity to CTX, both in vitro and in vivo.
- Overexpression of AXL in CTXS HNSCC lines rendered them resistant, whereas a kinase dead AXL retained sensitivity.
- Proteomic analysis within CTXR models indicated a strong linkage between AXL, Src Family Kinase (SFK) and HER3 activity, which is a novel pathway leading to therapeutic escape from CTX therapy driven by AXL in HNSCC.
We therefore hypothesize that AXL plays an important role in HNSCC pathogenesis and resistance to CTX and that AXL mediates resistance by activating the HER3/PI3K/Akt axis via SFKs. Therefore, targeting AXL has the potential to inhibit HER3/PI3K/AKT signaling and re-sensitize tumors to CTX therapy (Fig. 1). To test this hypothesis we propose the following aims:
- AIM 1: Determine A) how AXL activates the HER3/PI3K/Akt pathway in HNSCC and B) if AXL mediated activation of the HER3/PI3K/Akt pathway leads to CTX resistance. Preliminary data from our laboratory indicates that genetic ablation or therapeutic inhibition of AXL results in the down regulation of HER3 and SFK activity, suggesting that AXL may transmit signals to HER3, via SFKs, to manifest resistance. We will perform mechanistic in vitro and in vivo studies, using shRNA, Crispr-Cas9 and mutational analysis to determine if the AXL/SFK/HER3 signaling node plays a direct role in CTXR.
- AIM 2: Determine if targeting AXL in mouse models of CTXR can enhance and re-sensitize tumors to CTX. To accomplish this goal we will utilize several HNSCC models, including established CTXR cell lines and HNSCC PDXs in orthotopic xenografts. These tumor models will be treated with 1) vehicle, 2) Anti-AXL antibody, 3) CTX or 4) Anti-AXL antibody plus CTX; tumor growth will be used as the primary endpoint. Secondary endpoints will include tumor proliferation markers (Ki67), apoptosis (Caspase) and HER3 activity. These experiments will determine whether targeting AXL is a viable therapeutic approach and will identify potential predictive markers to be tested in Aim 3 and in future clinical studies.
- AIM 3: Determine A) whether AXL predicts resistance to CTX in HNSCC patients and B) if targeting AXL in CTXR PDXs enhances tumor response to CTX therapy. To translate these findings to humans, we will utilize biopsies from HNSCC patients to assess AXL and Ki67 proliferation index. Using a window-of-opportunity clinical trial, patients will be treated with CTX prior to surgery (window). To assess response to CTX, the diagnostic biopsy Ki67 score will be quantified and compared to the Ki67 score in the surgically resected tumor post CTX therapy and correlated with their AXL score (A). Further, we will expand PDXs from resistant patients to determine whether AXL blockade can overcome CTXR (B).