Co-leader: David Beebe, PhD
Professor of Pathology and Laboratory Medicine
University of Wisconsin

Co-leader: Paul Harari, MD
Professor of Human Oncology
University of Wisconsin
Summary:
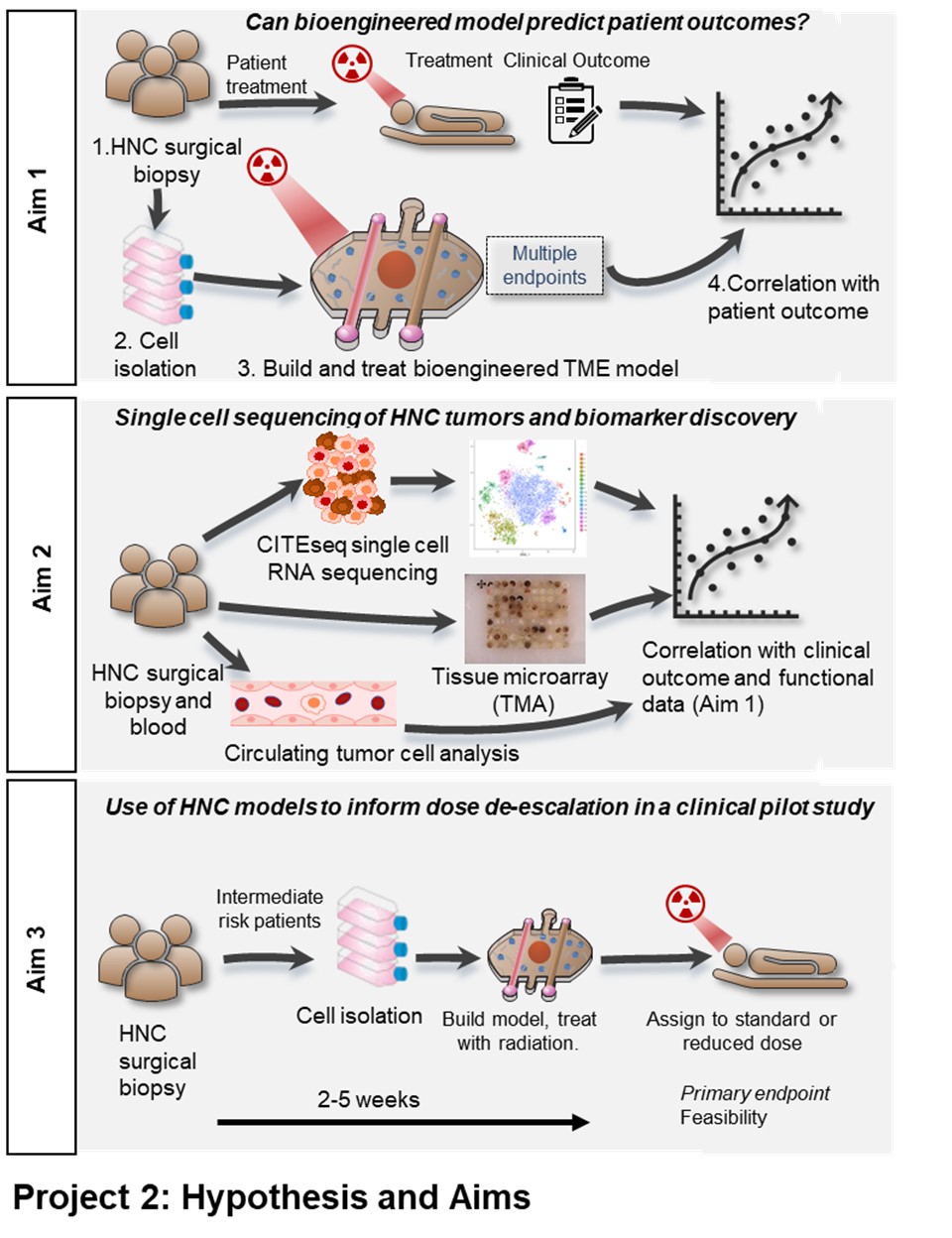
Our current inability to accurately predict treatment outcomes for head and neck cancer (HNC) patients represents a major challenge for clinicians and undoubtedly contributes to both the poor overall and progression free survival rates in advanced disease. Currently no predictive biomarkers are used clinically for definitive therapies, thus, there is a compelling need to develop new biomarkers and tools to improve clinical decision making. Functional biomarkers that can provide multiple orthogonal endpoints are well suited to reporting on a complex and dynamic environment such as the tumor microenvironment (TME). For this reason, we intend to investigate HNC biomarkers both directly in tumor samples and in a bioengineered patient-specific model to create a novel suite of endpoints. We will use state of the art single cell RNA sequencing (scCITE-seq), protein expression signatures from tumor tissue microarrays (TMA’s) and analysis of circulating tumor cells (CTC’s). We will utilize this multi-omic, patient-specific data set to identify and validate signatures of treatment efficacy and stratify patient outcomes. We will then test the feasibility of using patient specific bioengineered models to inform patient care in a clinical pilot study. The bioengineered model of the HNC TME is made entirely of cells derived from the same patient tumor sample, from the same patient cohort used for CITEseq, TMA and CTC analysis. These microscale patient-specific (built from the individual patients own cells) bioengineered models recapitulate the TME architecture, containing a HNC epithelial spheroid surrounded by a matrix containing fibroblasts and immune cells and flanked by blood and lymphatic microvessels. Our specific aims are: 1) Evaluate the ability of HNC patient-specific bioengineered models to predict treatment efficacy, where we will build patient-specific bioengineered models for 22 HNC patients (representing HPV-positive and HPV-negative disease and patients treated with primary surgery with (chemo)radiation or primary chemoradiation) and treat them with the same treatment the patient receives. Metrics of treatment success in the models will be correlated with actual patient outcomes including progression free survival. 2) Identify HNC biomarkers using scCITE-seq and TMA, where we will perform scCITE-seq and will correlate gene expression and cell populations with patient outcomes to investigate existing putative biomarkers and identify additional novel biomarkers. Biomarkers will be further investigated in a TMA and in CTC’s. 3) Use of bioengineered models to inform dose de-escalation in a clinical pilot study, where tissue will be acquired from surgery from 24 HPV+ HNC patients and used for patient-specific bioengineered model creation. Models will be treated to determine the radiosensitivity of a patient’s tumor and to stratify intermediate risk patients between 50 or 60 Gy treatment groups. Primary endpoints will focus on feasibility of model integration with secondary endpoints including local control. The successful completion of these aims will provide powerful new tools for the stratification of HNC patients and improved clinical decision making to help inform the most effective treatment selections for individual HNC patients in the future
Specific Aims:
-
AIM 1: Evaluate the ability of HNC patient-specific bioengineered models to predict treatment efficacy.
-
AIM 2: Identify HNC biomarkers using scCITE-seq and TMA.
-
AIM 3: Use of bioengineered models to inform dose de-escalation in a clinical pilot study.
Translational Significance
This project will use patient-specific bioengineered models, scCITE-seq and TMAs to advance our ability to predict treatment responses and thereby improve future clinical decision-making for HNC patients.
Collaborators:

Sheena Kerr, PhD
Research Assistant Professor
Carbone Cancer Center
University of Wisconsin

Adam Burr, MD, PhD
Assistant Professor
Department of Human Oncology
University of Wisconsin
Learn more: